Regulating Cardiac Contractile Force
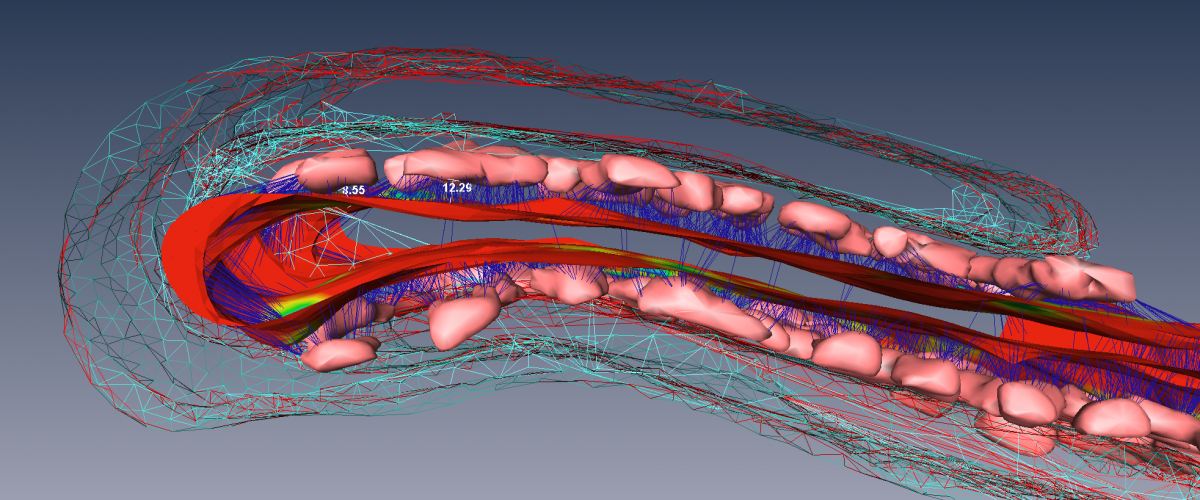
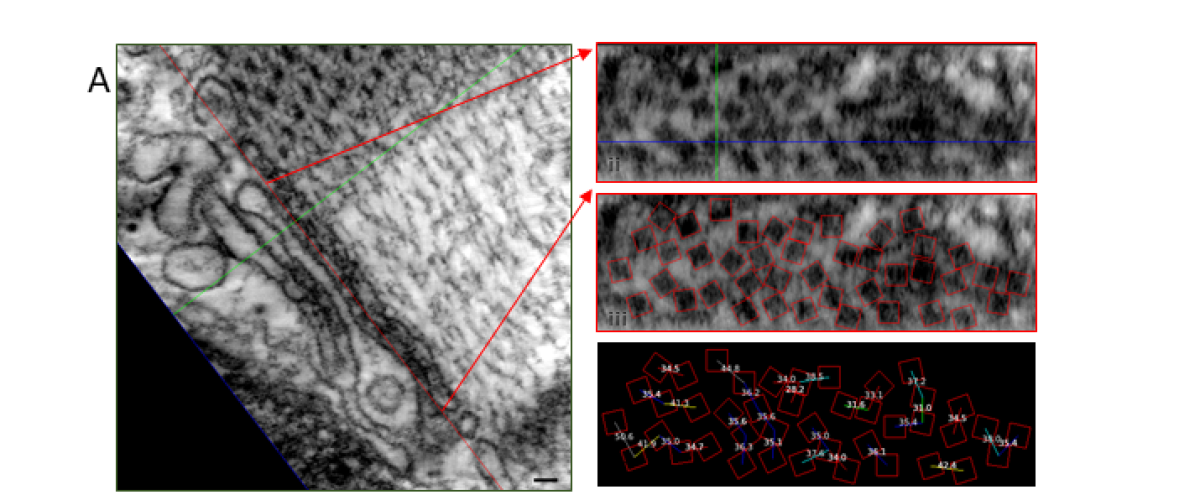
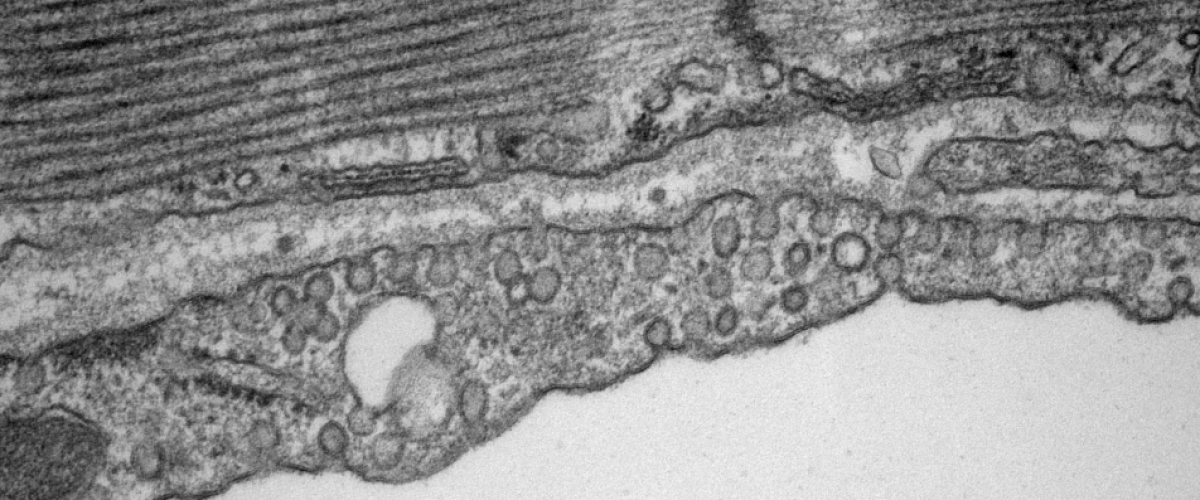
The type-2 cardiac ryanodine receptor (RyR2) is a Ca2+-activated Ca2+ ion channel located in the junctional sarcoplasmic reticulum (jSR) whose primary function is to regulate the amount, and the rate, of Ca2+ released from the jSR. Ca2+ release is the primary determinant of cardiac contractile force. Each jSR is decorated with between one and several hundred RyR2 that affect each other’s open probability through Ca2+-induced Ca2+ release (CICR) and through allosteric interactions. Recent and exciting data from the lab has shown that RyR2 channels are mobile and can interact with their neighbours in multiple ways. The interactions are dynamic and dependent on post-translational modifications and ligands. This opens an unprecedented higher-level type of regulation, whereby the activity of RyR2, and hence the amount of Ca2+ released, could be dictated by making and breaking allosteric coupling between RyR2 channels.
We are investigating RyR2 position and function in both normal and diseased tissue using a variety of cutting-edge and established techniques, including transmission electron microscopy, electron tomography, confocal and deconvolution microscopy, 3D superresolution immunofluorescence microscopy, transgenic mice, Ca2+ spark and Ca2+ transient analyses as well as biochemical approaches.